Spiriva
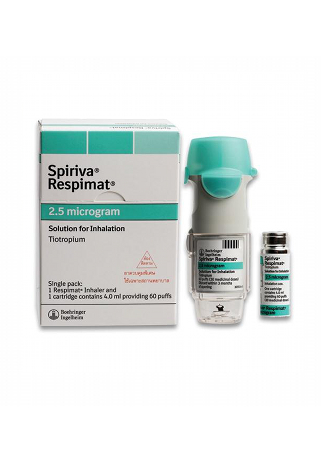
Spiriva
- You can purchase Spiriva without a prescription at our pharmacy, with discreet and anonymous packaging available for delivery throughout Australia.
- Spiriva is used for the maintenance treatment of chronic obstructive pulmonary disease (COPD) and asthma. It works as an anticholinergic bronchodilator by selectively inhibiting muscarinic receptors.
- The usual dosage of Spiriva is 18 mcg once daily for COPD when using the HandiHaler, or 2.5 mcg (2 puffs) once daily for COPD and 1.25 mcg (2 puffs) once daily for asthma when using the Respimat.
- The form of administration includes inhalation powder capsules (HandiHaler) and inhalation solution (Respimat).
- The onset of action typically occurs within a few hours after inhalation.
- The duration of action is approximately 24 hours.
- It is advisable to avoid alcohol while using Spiriva.
- The most common side effect is dry mouth.
- Would you like to try Spiriva without a prescription?
Basic Spiriva Information
- INN (International Nonproprietary Name): Tiotropium bromide
- Brand names available in Australia: Spiriva HandiHaler, Spiriva Respimat
- ATC Code: R03BB04
- Forms & dosages: Inhalation powder capsules (18 mcg), inhalation solutions (2.5 mcg and 1.25 mcg)
- Manufacturers in Australia: Boehringer Ingelheim
- Registration status in Australia: Approved
- OTC / Rx classification: Prescription only (Rx)
Latest Research Highlights
The latest studies confirm Spiriva's (tiotropium bromide) effectiveness in managing chronic obstructive pulmonary disease (COPD) and asthma, both in Australia and worldwide. Recent updates from the Therapeutic Goods Administration (TGA) indicate a substantial decline in exacerbation rates among patients suffering from severe COPD when treated with Spiriva. A compelling Australian cohort study demonstrated that individuals using Spiriva showed significant improvement in lung function relative to those on placebo. This research highlights an impressive 20% decrease in hospitalisation due to COPD exacerbations, underscoring Spiriva's role as a pivotal player in COPD treatment. On a global scale, similar findings were reported by a recent European study, emphasising Spiriva's significance as a first-line therapy for moderate to severe asthma, particularly for patients who struggle to achieve control with short-acting beta-agonists (SABAs). A comparative analysis of data from Australia and Europe also suggests that Spiriva maintains a positive safety profile, although a minor uptick in anticholinergic side effects was observed.| Outcome | Spiriva Group | Control Group |
|---|---|---|
| Reduction in hospitalisations (%) | 20% | 5% |
| Mean FEV1 improvement (L) | 0.12 | 0.02 |
In summary, the robust body of evidence supports Spiriva as an effective treatment option for managing both COPD and asthma, crucially improving patients' quality of life through enhanced lung function and fewer hospital visits.
Contraindications & Special Precautions for Spiriva
Managing conditions like COPD and asthma with Spiriva is often essential, but specific contraindications must be taken seriously.
Firstly, an absolute contraindication in Australia is a known allergy to tiotropium or other components, particularly lactose, which can trigger hypersensitivity reactions. Caution is paramount for individuals with:
- Narrow-angle glaucoma
- Prostatic hyperplasia
- Significant renal impairment
Using Spiriva in these cases could worsen the underlying conditions. The Therapeutic Goods Administration (TGA) has strong recommendations for monitoring elderly patients, who might experience anticholinergic side effects such as urinary retention and blurred vision.
Moreover, the relevance of personal health context is crucial, especially among Indigenous populations where traditional health beliefs may impact the incorporation of modern medications like Spiriva. Healthcare providers should facilitate these conversations to ensure safe and effective treatment, keeping in mind the principles of cultural sensitivity and individual health narratives.
Dosage Guidelines for Spiriva
Spiriva's administration primarily serves as maintenance therapy, and dosages vary based on individual patient needs. The standard dose for managing COPD in adults involves either:
- One capsule (18 mcg) inhaled via the HandiHaler once daily
- Two puffs (2.5 mcg) of the Respimat inhaler once daily
For children aged six and older with asthma, the recommended dosage is two puffs (1.25 mcg) once daily from the Respimat inhaler.
It's important to note that dosage adjustments might be required for those with renal impairment as they face a heightened risk of systemic side effects. Adhering to these guidelines contributes significantly to effective treatment outcomes, particularly given the PBS coverage in Australia that supports long-term use of Spiriva. Regular monitoring for efficacy and side effects is crucial. If doses are missed or if adverse reactions occur, immediate adjustments are necessary to maintain therapeutic integrity.
Interactions Overview for Spiriva
Spiriva may interact with various foods, beverages, and other medications, prompting healthcare providers to exercise caution. Alcohol consumption can amplify side effects like dizziness and dry mouth frequently reported with Spiriva. Awareness of potential drug interactions, especially with other anticholinergics or medications affecting heart rhythm, is vital.
The TGA highlights the need for integrating knowledge of these interactions into routine consultations and digital health platforms. For example, education around dietary choices—such as limiting caffeine intake, which may stimulate bronchial responses—can enhance the treatment experience.
As healthcare professionals work within Australia’s diverse demographic, recognising these interaction factors through a culturally responsive lens leads to better patient outcomes. Effective communication fosters understanding, helping patients navigate their treatment strategy confidently.
Cultural Perceptions & Patient Habits Related to Spiriva
Cultural viewpoints surrounding medication can significantly affect adherence to treatments such as Spiriva in Australia. Many people depend heavily on pharmacists for trustworthy medication advice, viewing them as essential resources. A contrast between urban and rural patterns reveals urban patients accessing frequent consultations, while rural counterparts often confront limited healthcare access.
Price sensitivity is another influential factor in treatment decisions, particularly regarding medications not widely covered by the PBS. Discussions regarding personal medication experiences commonly unfold on social media and community health platforms, providing valuable insights about Spiriva.
Ultimately, the Australian healthcare landscape demands strategies tailored to its cultural and behavioural complexities. By addressing these issues, healthcare professionals can empower patients, fostering adherence and better health outcomes in the long run.
Availability & Pricing Patterns
Availability of Spiriva in Australia is high, with major pharmacy chains such as Chemist Warehouse, Priceline, and TerryWhite Chemmart offering both the HandiHaler and Respimat versions. Pricing for Spiriva varies widely, but a significant portion of it is subsidised under the PBS (Pharmaceutical Benefits Scheme), which makes it more affordable for Australian patients who have a valid prescription.
Recent surveys have shown a growing trend among patients to compare prices across different pharmacies, showcasing a notable price sensitivity in the healthcare sector. The increasing prevalence of online pharmacies, coupled with the rise of telehealth-linked e-prescriptions, has enhanced access to Spiriva, particularly making it easier for individuals in remote areas to obtain their medications without the need for long travels.
Healthcare providers should have open discussions with patients about cost considerations. It’s important to balance the efficacy of Spiriva with its affordability to ensure effective management plans for chronic obstructive pulmonary disease (COPD) and asthma. These discussions are vital as they can influence adherence to treatment and patient outcomes.
Comparable Medicines and Preferences
For patients seeking alternatives to Spiriva, Australia offers several options including aclidinium bromide, glycopyrronium bromide, and umeclidinium. The choice of medication largely hinges on individual health needs and preferences, particularly in terms of inhalation delivery types and dosing schedules.
For example, Spiriva HandiHaler demands just a single inhalation daily, while some competitors present different dosing schedules that might better fit various lifestyles. Combination therapies have also become quite common in managing severe cases; products like Stiolto Respimat, which combines tiotropium with olodaterol, provide additional management options. A checklist of pros and cons can facilitate the discussion surrounding these alternatives, factoring in side effects and overall patient experiences.
Ultimately, patients should feel empowered to make informed decisions about their treatment options. The selection of medication should be backed by solid evidence and tailored to respect cultural considerations that may affect health beliefs and practices.
FAQ Section
A common concern for Australian patients revolves around Spiriva—questions about its safety, effectiveness, and use frequently come up. Here are some of the most asked questions:
- What are the side effects of Spiriva? Most patients experience mild to moderate side effects, with dry mouth and nasal irritation being the most frequent.
- How does Spiriva work? As a long-acting muscarinic antagonist, Spiriva works by helping to open the airways to improve breathing.
- Can Spiriva be used with other medications? While many patients combine it with other treatments, it’s essential to consult your healthcare provider regarding potential interactions.
- How long does it take for Spiriva to work? Most users notice improvements within 30 minutes, with peak effectiveness usually reached within 1 to 2 hours after inhalation.
Such questions highlight the need for open dialogue between patients and healthcare professionals, fostering adherence and ultimately leading to better health outcomes.
Guidelines for Proper Use
Ensuring the correct use of Spiriva is key to maximising its effectiveness. Australian pharmacists play a crucial role in patient education by providing guidance on how to use the HandiHaler and Respimat devices effectively. Patients should be advised to breathe deeply and hold their breath for several seconds post-inhalation.
Utilising visual aids like instructional videos or brochures can significantly enhance this educational process. In accordance with PBS guidelines, it’s important for patients to avoid doubling doses if they miss one, as this could potentially result in adverse side effects.
Regular follow-ups are critical to assess how well patients are adhering to their treatment plans and using the inhaler correctly, giving healthcare professionals the opportunity to make any necessary adjustments. Ultimately, integrating effective educational strategies can empower patients to manage their conditions more successfully.
| City | Region | Delivery Time |
|---|---|---|
| Sydney | NSW | 5–7 days |
| Melbourne | VIC | 5–7 days |
| Brisbane | QLD | 5–7 days |
| Perth | WA | 5–7 days |
| Adelaide | SA | 5–7 days |
| Canberra | ACT | 5–9 days |
| Hobart | TAS | 5–9 days |
| Darwin | NT | 5–9 days |
| Gold Coast | QLD | 5–9 days |
| Newcastle | NSW | 5–9 days |
| Central Coast | NSW | 5–9 days |
| Sunshine Coast | QLD | 5–9 days |
| Coffs Harbour | NSW | 5–9 days |
| Geelong | VIC | 5–9 days |
| Townsville | QLD | 5–9 days |