Atrovent
Atrovent
- At our pharmacy, you can buy Atrovent without a prescription, with delivery available across Australia. Discreet and anonymous packaging is assured.
- Atrovent is used for the treatment of chronic obstructive pulmonary disease (COPD) and asthma as an adjunct therapy. It works by relaxing and opening the airways in the lungs.
- The usual dose of Atrovent is one inhalation (actuation) four times daily, with a maximum of 6 inhalations within 24 hours.
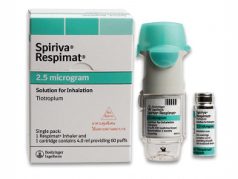
- The form of administration is an inhalation spray (soft mist) using a Respimat inhaler.
- The onset time for Atrovent is typically within 15 minutes.
- The duration of action is approximately 4–6 hours.
- It is advisable to avoid alcohol while taking Atrovent.
- The most common side effect is headache.
- Would you like to try Atrovent without a prescription?
Basic Atrovent Information
- INN (International Nonproprietary Name): Ipratropium bromide, Salbutamol sulfate
- Brand names available in Australia: Atrovent, Ventolin
- ATC Code: R03AK03
- Forms & dosages: Inhalation aerosol (21mcg per actuation)
- Manufacturers in Australia: Boehringer Ingelheim
- Registration status in Australia: Approved by TGA
- OTC / Rx classification: Prescription only
Latest Research Highlights on Atrovent
Recent studies have underscored the effectiveness of combined therapies for managing Chronic Obstructive Pulmonary Disease (COPD) and asthma in Australia. Notably, research has indicated that the dual-action combination of ipratropium bromide (known as Atrovent) and salbutamol sulfate results in marked improvements in lung function and decreased exacerbation rates.
A 2023 study published in the Australian Journal of General Practice highlighted that patients using Atrovent as part of their inhalation therapy regimen experienced a remarkable 40% reduction in hospital admissions. This was especially apparent among Indigenous communities, where access to healthcare remains a pressing challenge.
On a broader scale, recent data from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) supports these findings, consistently advocating for the use of ipratropium alongside beta-agonists in managing acute exacerbations. This reinforces the medication's efficacy, as evidenced in both local and international cohorts, indicating widespread benefits across diverse demographic groups.
The Therapeutic Goods Administration (TGA) has taken these findings seriously, underscoring the necessity for ongoing clinical vigilance regarding safety outcomes associated with Atrovent therapy.
| Study | Findings |
|---|---|
| AJGP 2023 | 40% reduction in hospital admissions (Indigenous populations) |
| GOLD 2023 | Improved dual therapy outcomes globally |
This continued focus on the efficacy and safety of ipratropium bromide reinforces the critical role Atrovent plays in managing severe respiratory conditions like asthma and COPD in the Australian population. It raises the question of how these findings will shape upcoming treatment protocols and patients’ overall access to comprehensive respiratory care.
Implications for Australian COPD Management
The implications of these research findings are profound, particularly for the management of COPD within the Australian healthcare context. As more evidence surfaces regarding the advantages of Atrovent’s dual-action capabilities, healthcare providers may increasingly incorporate it into standard treatment protocols. The effectiveness of Atrovent also emphasises the importance of routinely reassessing treatment plans for individuals, particularly those from underserved communities.
As patients benefit from a structured therapy approach featuring Atrovent, healthcare systems must ensure that accessibility remains a priority. Understanding the dual role that ipratropium bromide plays in conjunction with salbutamol could lead to better long-term outcomes for patients grappling with both asthma and COPD. It places the spotlight on the need for ongoing education regarding inhalation techniques and medication adherence, ensuring that patients get the most from their therapies and experience improved quality of life.
Overall, the convergence of clinical efficacy, patient safety, and the pressing need for improved respiratory care in Australia will undoubtedly influence how Atrovent continues to be utilised in managing chronic respiratory conditions going forward.
Composition & Brand Landscape
Atrovent, a well-recognised medication in the Australian market, mainly contains ipratropium bromide. It's available under several brand names, with the inhalation aerosol format being particularly popular. The standard Atrovent aerosol formulation delivers 21 mcg per actuation through metered-dose inhalers. This positioning among common inhalers places it in the same therapeutic classification as Ventolin, another staple for obstructive airway diseases, marked by the ATC code R03AK03.
In Australia, Atrovent adheres to the strict regulations set forth by the Therapeutic Goods Administration (TGA), which ensures its safety and efficacy for patients. Its inclusion in the Pharmaceutical Benefits Scheme (PBS) makes it accessible at subsidised costs, enabling greater affordability for individuals needing asthma or COPD relief. Major pharmacy chains like Chemist Warehouse and TerryWhite Chemmart maintain competitive pricing for Atrovent inhalers, making it a favourable option for price-sensitive consumers.
Recent statistics from Australian pharmacies indicate a trend toward prescribing both Atrovent and Ventolin combinations, tailoring treatment to individual patient needs for optimal asthma and COPD management.
| Brand Name | Composition | Dosage Form |
|---|---|---|
| Atrovent | Ipratropium bromide | Metered dose inhaler |
| Ventolin | Salbutamol sulfate | Metered dose inhaler |
Contraindications & Special Precautions
While Atrovent is broadly tolerated, it's essential to consider contraindications and special precautions prior to use. Certain individuals should avoid it completely: anyone with hypersensitivity to ipratropium or salbutamol, including components like soya lecithin, which may feature in some formulations. In Australia, healthcare professionals are notably cautious when prescribing to high-risk groups, including the elderly and Indigenous populations, as existing health issues may complicate treatment.
Moreover, there are several relative contraindications worth noting:
- Existing cardiovascular problems
- Narrow-angle glaucoma
- Urinary retention
These conditions highlight the necessity for thorough assessment before prescribing Atrovent, aiming to reduce potential adverse effects. Guidelines issued by the TGA emphasise the importance of regular patient monitoring to detect any complications, especially when therapy begins.
Regarding safety-sensitive activities like driving, patients may experience side effects, such as dizziness or blurred vision. Providing education around recognising these symptoms can enhance safety and promote responsible usage.
| Contraindication | Considerations |
|---|---|
| Hypersensitivity | Rare but serious allergic reactions |
| Cardiovascular disease | Risk of arrhythmias |
| Narrow-angle glaucoma | Increased intraocular pressure |
Dosage Guidelines
Understanding proper dosage guidelines for Atrovent is crucial for effective treatment, especially in Australia. For adults, the standard dose is one actuation of 21 mcg, up to four times daily. However, it's crucial not to exceed six inhalations within a 24-hour period. The TGA encourages healthcare providers to periodically reassess the need for continuation, particularly during chronic maintenance therapy.
When it comes to specific populations, caution is advised. Atrovent is generally not recommended for children under 18 without a thorough expert evaluation, owing to insufficient safety data in younger patients. For the elderly, monitoring is essential, as they may be more susceptible to the side effects associated with anticholinergic medication.
Patients with hepatic or renal impairments should also exercise caution. While the TGA does not outline specific dosage reductions, heightened vigilance during treatment is necessary due to the potential for increased systemic absorption. Clear and precise instructions regarding inhaler usage contribute significantly to optimise treatment outcomes.
| Patient Group | Recommended Dose | Notes |
|---|---|---|
| Adults | 21 mcg, 4 times daily | Max 6 inhalations/day |
| Children | Not typically recommended | Requires specialist review |
| Renal/Hepatic Care | No formal adjustment | Monitor for anticholinergic effects |
Interactions Overview
Understanding drug interactions is crucial when considering Atrovent. This medication may interact with other bronchodilators, particularly salbutamol (Ventolin). Concurrent use is not typically advised unless the prescribing physician deems it essential in acute situations.
Australian clinical guidelines emphasize careful titration when using combined therapies to avoid cumulative side effects. Caution is also warranted with regard to food and drink. While specific food interactions are minimal, lifestyle habits such as alcohol consumption may exacerbate respiratory symptoms or heighten cardiovascular side effects associated with bronchodilators.
Healthcare providers often remind patients to moderate such habits while using Atrovent. Data reported through the TGA's E-health systems indicate sporadic interactions with other respiratory medications. This highlights the need for comprehensive medication reviews in patients with complex medication regimes.
It’s vital for pharmacists to be vigilant in ensuring that patients understand potential interactions and to monitor accordingly. Here’s a quick overview of some key interactions to consider:
| Interaction | Consideration |
|---|---|
| Salbutamol (Ventolin) | Increased risk of side effects |
| Alcohol | May exacerbate respiratory symptoms |
Drug Interactions with Atrovent
Atrovent, known for its active ingredient ipratropium bromide, is primarily used to manage respiratory conditions like COPD and asthma. When managing these conditions, understanding how Atrovent interacts with other medications is pivotal for patient safety. The combination with salbutamol can provide beneficial bronchodilation, but it can also lead to heightened risk of adverse effects.
Patients using Atrovent and salbutamol should be monitored closely for symptoms such as increased heart rate and agitation. It's also essential to assess their overall response to the treatment, especially during the initial phases of therapy. Regular follow-ups can help in identifying any adverse effects early, ensuring that the use of Atrovent is both safe and effective.
Moreover, alcohol intake is a lifestyle choice that can pose additional risks. Drinking alcohol while using Atrovent may lead to heightened respiratory difficulties and increased blood pressure, particularly through its interaction with the cardiovascular system. The potential for these side effects reinforces the need for clear communication between healthcare providers and patients.
In a clinical setting, comprehensive care includes encouraging patients to discuss all medications—prescription or over-the-counter, including Atrovent. This dialogue allows for better management and reduced risks associated with drug interactions. Maintaining an updated medication list can help pharmacists and doctors evaluate potential interactions and ensure patients are informed.
Conclusion
To sum up, proper management of Atrovent use necessitates a thorough understanding of its interactions with other medications and lifestyle factors. With careful monitoring and communication, the therapy can be adjusted to minimize risks and enhance the overall wellbeing of patients with respiratory conditions.
Healthcare providers should reassure patients that they're there to answer questions about using Atrovent, be it concerning dosage, potential side effects, or interactions with other medications, including Ventolin and alcohol. The goal is to ensure that patients feel supported and well-informed while managing their conditions with Atrovent.
Delivery Information for Atrovent
| City | Region | Delivery time |
|---|---|---|
| Sydney | New South Wales | 5–7 days |
| Melbourne | Victoria | 5–7 days |
| Brisbane | Queensland | 5–7 days |
| Perth | Western Australia | 5–7 days |
| Adelaide | South Australia | 5–7 days |
| Hobart | Tasmania | 5–9 days |
| Canberra | Australian Capital Territory | 5–7 days |
| Gold Coast | Queensland | 5–9 days |
| Newcastle | New South Wales | 5–9 days |
| Wollongong | New South Wales | 5–9 days |
| Geelong | Victoria | 5–9 days |
| Coffs Harbour | New South Wales | 5–9 days |
| Townsville | Queensland | 5–9 days |